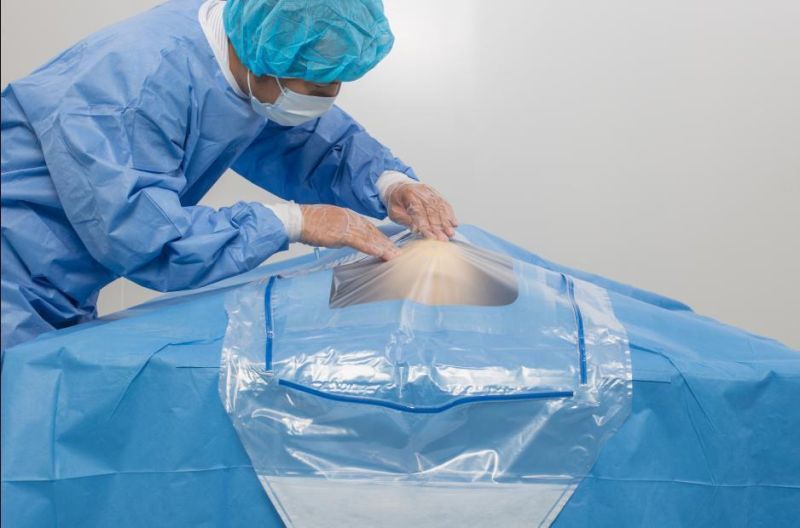
Eo Sterile Non Woven Surgical Craniotomy Drape / Craniotomy Drape Surgical Drape Suppliers with CE ISO13485 Certification
Henan Joinkona Medical Products Stock Co., Ltd.- Material:PP
- Feature:Disposable
- Certification:CE, FDA, ISO13485
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Application:Hospital
- Group:Patients with Craniocerebral
Base Info
- Model NO.:23*305cm
- Logo Printing:Without Logo Printing
- Transport Package:Individual Packing, 50PCS,CTN
- Specification:SMMS,SPP
- Trademark:JOINKONA, OEM
- Origin:China
- HS Code:6307900090
- Production Capacity:50000PCS,Day
Description
Basic Info.
Model NO. 23*305cm Logo Printing Without Logo Printing Transport Package Individual Packing, 50PCS/CTN Specification SMMS/SPP Trademark JOINKONA, OEM Origin China HS Code 6307900090 Production Capacity 50000PCS/DayProduct Description
Products: Craniotomy drape
Measure: Regular size 230x305cm or customizedMaterial: Could be made with SMS, Bi-SPP Lamination fabric, Tri-SPP Lamination fabric, Bi-Viscose Lamination fabric, Tri- Viscose
Lamination fabric, PE film, SS
Feature: Disposable and EO sterilizable
Usage: It is used in the operation room for neuro surgery, the product is no irritation, no toxicity and inodorous to human,there is no energy and no side effect to body. Can be used to improve the ease, efficiency and safety of surgical procedure, mean while Reduced the risks of accidental cross infection
Packaging: Individual packed in paper&plastic sterile bag, or customized packing eved through impermeable materials where needed,while effective fluid control is obtained through absorption or fluid collection pouches allowing for a drier working area.