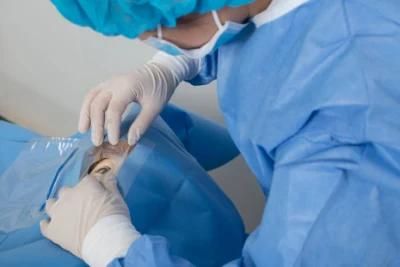
Eo Sterile Non Woven Surgical Ophthalmic Drape / Ophthalmic Surgical Drape Suppliers with CE ISO13485 Certification
Henan Joinkona Medical Products Stock Co., Ltd.- Material:PP
- Feature:Disposable
- Certification:CE, FDA, ISO13485
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Application:Hospital
- Logo Printing:Without Logo Printing
Base Info
- Model NO.:100*130CM
- Transport Package:Individual Packing, 50PCS,CTN
- Specification:PP, PP +PE, SMS, etc
- Trademark:JOINKONA, OEM
- Origin:China
- HS Code:6307900090
- Production Capacity:50000PCS,Day
Description
Basic Info.
Model NO. 100*130CM Transport Package Individual Packing, 50PCS/CTN Specification PP, PP +PE, SMS, etc Trademark JOINKONA, OEM Origin China HS Code 6307900090 Production Capacity 50000PCS/DayProduct Description
Product Description
Item Name: Ophthalmic Drape Material SMS/SPP
Size: 100*130cm or customized
Color: Blue or customized
Packing :1pc/pouch , 25pcs/box. 100PCS/carton
Carton Size:50*30*40cm
Supply Ability: 2000000/pieces month
Loading Port: Qingdao
OEM Offered
Delivery Time: About 25-45 days, based on order quantity
Certification: ISO 13485, CE
Applications:
This drape is used in the operation room for Ophthalmid Surgery, It can be used to improve the convenience, efficiency and safety of surgical procedures, meanwhile reducing the risks of accidental cross infection.
Joinkona ophthalmic drapes are available in a variety of sizes with different design features to meet the needs and surgery requirements of ophthalmic surgeons worldwide.
