Disposable Skin Stapler
Changzhou Medical Bioengineering Co., Ltd.- Material:ABS,Stainless Steel
- Feature:Disposable
- Certification:CE, ISO13485
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Application:Hospital
- Group:All
Base Info
- Model NO.:35W, 35R
- Logo Printing:With Logo Printing
- Transport Package:Pet Box + Tyvek Paper
- Specification:35W, 35R
- Trademark:CYS
- Origin:China
- HS Code:9018909919
- Production Capacity:40, 000PCS,Month
Description
Less than 5 mm
Ankle, Knee, HeadCSPF-25R 5.4×3.5 mm 25 pcs CSPF-CW CSPF-35R 5.4×3.5 mm 35 pcs CSPF-CW 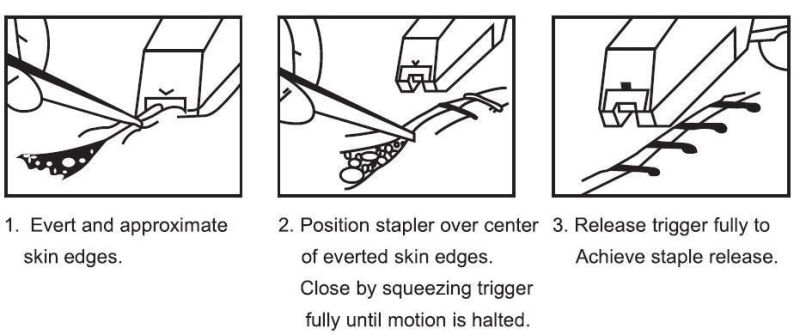
Hospital Departments
General surgical operation, Sardiothoracic surgery, Plastic surgery, Neurosurgery operation, Gynecologic surgery, Burn surgery, Emergency operation and Field rescue.
Postoperative Effect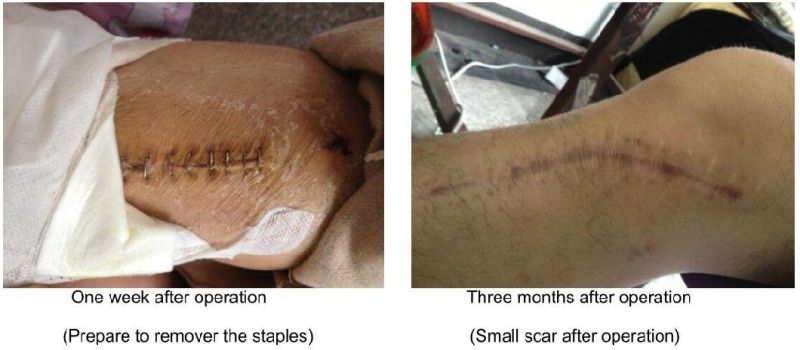
Attention:
It is not suitable for the incision of the operation, which is required for docimasia after operation.
It is prohibited to use the disposable skin stapler in suture of interior tissues.
The product is prohibited to use when the package is broken.
The product is prohibited to use when it exceed the valid date.
The product is sterilized and discard for single use, repeat use is prohibited.
The operation doctor should be trained.
Available Certificates:
Ankle, Knee, Head

Hospital Departments
General surgical operation, Sardiothoracic surgery, Plastic surgery, Neurosurgery operation, Gynecologic surgery, Burn surgery, Emergency operation and Field rescue.
Postoperative Effect

Attention:
It is not suitable for the incision of the operation, which is required for docimasia after operation.
It is prohibited to use the disposable skin stapler in suture of interior tissues.
The product is prohibited to use when the package is broken.
The product is prohibited to use when it exceed the valid date.
The product is sterilized and discard for single use, repeat use is prohibited.
The operation doctor should be trained.
Available Certificates:

