Hemoperfusion Cartridge (ESRD)
Guangdong Baihe Medical Technology Co., Ltd.- Type:Vacuum Blood Tube & Blood Bag
- Material:Plastic
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Three Years
- Group:Adult
- Logo Printing:With Logo Printing
Base Info
- Model NO.:MG150 MG250 MG350
- Transport Package:Carton
- Specification:49*41.5*22cm
- Origin:China
- HS Code:9013809000
- Production Capacity:50000000PCS,Year
Description
Basic Info.
Model NO. MG150 MG250 MG350 Transport Package Carton Specification 49*41.5*22cm Origin China HS Code 9013809000 Production Capacity 50000000PCS/YearProduct Description
HEMOPERFUSION CARTRIDGE INFORMATION
The combined hemodialysis/ hemoperfusion procedure (HD+HP) makes advantage of each other for blood purification, in this way, metabolic products and uremic toxins produced by renal failure patient could be thoroughly removed and the water, as well as electrolyte could be regulated to balance. Eventually, internal environment remains balance and patient's life quality is improved.End Stage Renal Disease (ESRD)
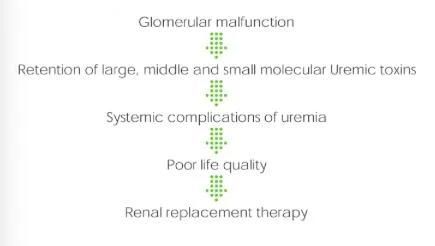
Hemoperfusion treatment sketch
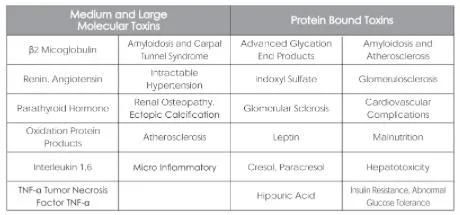
Some Toxins of Uremia and Relevant Complications
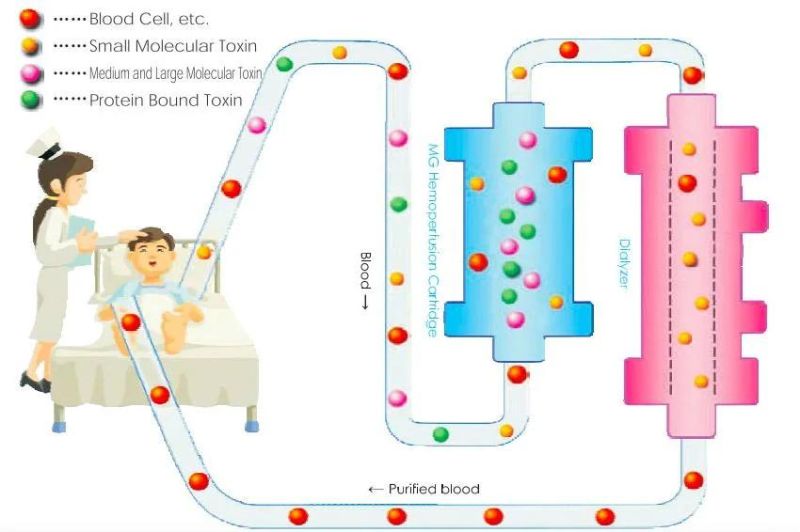
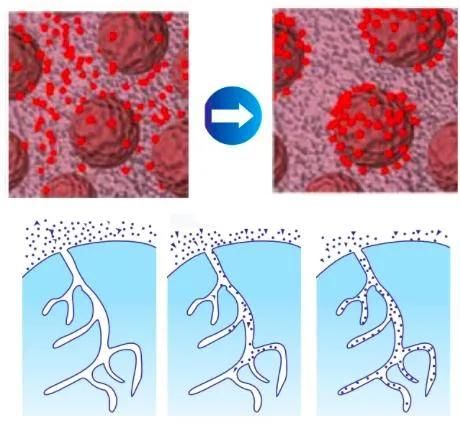
Adsorption Sketch of MG Series Hemoperfusion Cartridge
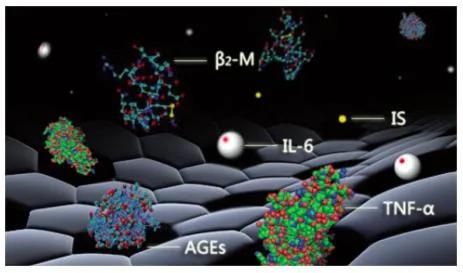
Poisoned
Hemoperfusion is to eliminate the endogenous and exogenous pathogenic substances so as to purify the blood by circulating the blood through the absorbed device extracorporeally. It can absorb effectively the fat-soluble medium and large molecular medicine and poison, and medicine and poison with high protein binding rate.Absorption Mechanism
HEMOPERFUSION CARTRIDGE BY BAIHE MEDICAL
ORDER INFORMATION