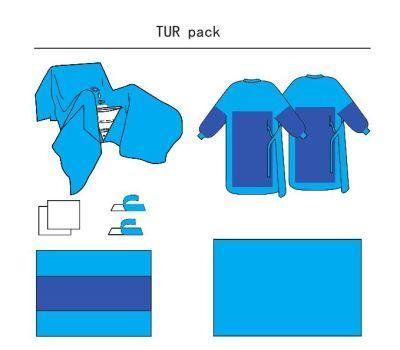
Disposable CE and ISO13485 Approved Sterile Tur Surgical Pack
Henan Joinkona Medical Products Stock Co., Ltd.- Feature:Disposable
- Certification:CE, FDA, ISO13485
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Application:Hospital
- Group:Urology Department Surgery Patients
- Logo Printing:Without Logo Printing
Base Info
- Model NO.:JK-TP-204
- Transport Package:1PC,Sterile Pouch, 6PCS,Carton
- Specification:PP, PP +PE, SMS, etc
- Trademark:Joinkona,OEM
- Origin:China
- HS Code:6307900090
- Production Capacity:200000PCS,Month
Description
TUR Surgical Pack
Code
Specification
- All the specification and contents can be customized
- Customized contents welcome
Label
Customized printing is available
Material
PP, PP +PE,SMS,etc
Packing
1pc/sterile pouch, 8pcs/carton
Carton Dimension
55*45*42cm
Sterile or not
Yes, EO(Ethylene Oxide)
Brand
Medical Technology/OEM is available
Usage of the Main Product Feature:
1.TUR drape: impede penetration of liquid and bacterial and effectively prevents cross infection when covering the patient.
2. OP tape:Used to fix the product and drapes during surgical procedures.Easy to past and remove.
