
High Biocompatible Medical Cobalt Chromium Coronary Cardiac Stent System
Fortala Laboratories Shenzhen Limited- Type:Catheter
- Material:Cobalt-Chromium
- Ethylene Oxide Sterilization:Ethylene Oxide Sterilization
- Quality Guarantee Period:Three Years
- Group:Adult
- Logo Printing:With Logo Printing
Base Info
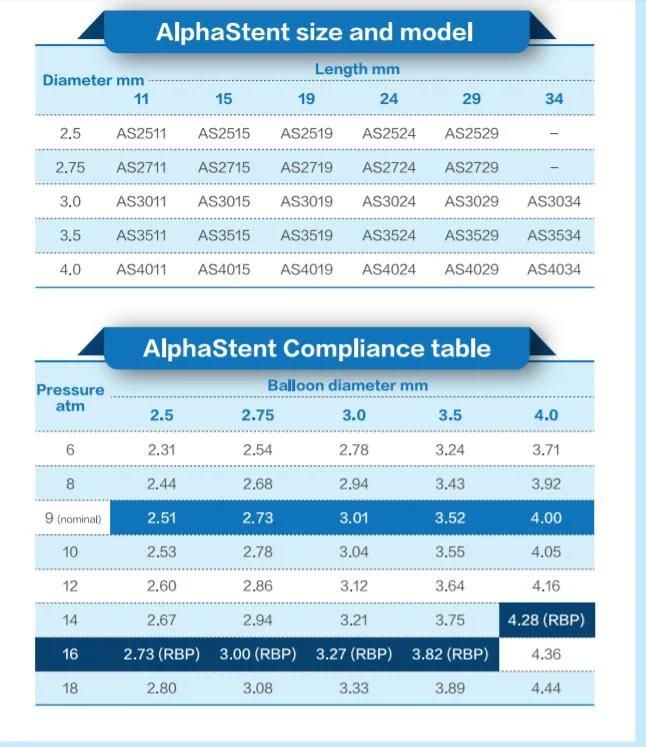
- Model NO.:AS-0011
- Use for:Interventional Cardiology
- Sterile:Sterilization
- Diameter:Diameter From 2.0mm to 5.0mm
- Transport Package:Box, Al Bag, Pouch
- Trademark:OEM
- Origin:Shenzhen China
- HS Code:90183900
- Production Capacity:50000pieces,Month
Description
Basic Info.
Model NO. AS-0011 Use for Interventional Cardiology Sterile Sterilization Diameter Diameter From 2.0mm to 5.0mm Transport Package Box, Al Bag, Pouch Trademark OEM Origin Shenzhen China HS Code 90183900 Production Capacity 50000pieces/MonthProduct Description
Product Indication:1. The residual stenosis after percutaneous transluminal coronary angioplasty is still greater than 30%.
2. Acute myocardial infarction.
3. Severe intimal tear or acute vascular blockage occurred during percutaneous coronary angioplasty.
Product Features:
- Patented high biocompatibility
- Fluoropolymer and drug carrier
- Thin strut thickness
- Decent hoop strength
- Excellent radiopacity
Product Description:
Coronary Stent, also known as heart stent, is a commonly used medical device in interventional heart surgery.
Coronary artery stent is an intravascular support made of metal stainless steel or other special materials. It has good plasticity and geometric stability. It can be delivered to the diseased site via a cardiac catheter in a closed state, and then expanded by balloon expansion and other methods to support the blood vessel wall. Coronary artery stents are also the main treatment for coronary heart disease.
Shipping:
Express, Air, sea shipping is available

